A Garden in Deep Freeze
Posted on Thu., May 12, 2016 by
The Huntington's cryopreservation program strives to conserve endangered plants

Raquel Folgado, a plant physiologist at The Huntington, dissects a magnolia shoot tip before conducting a cryopreservation experiment.
The caretakers of the tender succulents in the Desert Garden may cringe at news of a prolonged cold snap, but Raquel Folgado, a plant physiologist at The Huntington, thinks nothing of plunging aloe plants into temperatures 321 degrees Fahrenheit below zero.
In her lab near The Rose Hills Foundation Conservatory for Botanical Science, Folgado opens a flask of liquid nitrogen. As the ultracold liquid billows out clouds of vapor, she drops in tiny bits of sterile aloe. Folgado will later pull out the frozen bits of tissue, thaw them, and gently place them in petri dishes. She will then line the dishes up under a series of grow lamps. Through the lens of a Nikon camera mounted on her microscope, she'll watch over the next few weeks as the thawed dabs of plant tissue, like tiny green miracles, grow into rows of healthy new aloe plants.
Folgado is one of a small fraternity of researchers attempting to save plants by freezing them. Called plant cryopreservation, the young field uses still-developing and sometimes trial-and-error techniques to allow plant material to survive temperatures that normally would cause instantaneous death. The plant tissue can then be thawed and regrown when and where new plants are needed most.
Preserving plant genetic material, or germplasm, is a critical part of safeguarding the world's food supply, creating new varieties of plants, and being able to bring back plants that have been destroyed by disease, pests, and manmade or natural disasters. The backbone of this effort consists of large seed banks, kept in massive freezers or, in the case of Svalbard's "Doomsday Vault," in the belly of a frozen Arctic mountain. But seed banking doesn't work for plants that produce few or no seeds, plants that don't grow true from seed, or plants with seeds that can't be frozen. In these cases, cryopreservation of specialized plant tissue is necessary. But because the work is difficult, time-consuming, and exacting, it has mainly taken place in large government or internationally funded labs, and rarely at smaller institutions.
Huntington botanists have plunged into this emerging field because such work is considered urgent. Many conservationists believe that traditional methods of saving plants, like protecting habitats or growing rare plants in preserves, are no longer enough. "We're living in such an incredible age of extinction that we have to take extraordinary measures if we want to take care of our plants," says Peter Raven, president emeritus of the Missouri Botanical Garden and chair of the board of trustees of the Center for Plant Conservation. "The work The Huntington is doing is visionary."

Aloe fievetii is grown in sterile conditions before being dissected by Raquel Folgado for use in her cryopreservation protocol. Photograph by Kate Lain.
While the project is futuristic, the inspiration for it is nearly 100 years old. It's a set of thick black binders overflowing with yellowing pages that sits stacked outside the office of Jim Folsom, the Marge and Sherm Telleen/Marion and Earle Jorgensen Director of the Botanical Gardens at The Huntington. These carefully typed records document every plant that has ever graced the gardens. But when Folsom flips through the long lists of plants, he sees one word stamped in red capital letters next to most of the entries: "DEAD. DEAD. DEAD."
This loss is common for botanical gardens—plants don't live forever—and it sadly mirrors the decimation of plants worldwide. One in five plant species—and one in three cactus species—are currently at risk for extinction, according to recent estimates. "We're losing so much so fast," says Folsom. "I thought, if I could do one thing now to stop this disaster, what would it be?"
The answer came in 2013 when The Huntington's plant conservation specialist, Sean Lahmeyer, visited the U.S. Department of Agriculture's National Center for Genetic Resources Preservation in Fort Collins, Colo. The Fort Knox–like facility warehouses some 600,000 unique specimens of U.S. agricultural plants in vast freezers, an underground vault, and hundreds of vats of liquid nitrogen the size of Jacuzzis.
While Lahmeyer knew The Huntington's staff couldn't work on the same scale, he thought they could aid the global effort by cryopreserving their own plants. The start-up costs did not seem that high. "With our collection, we could work on so many things," he says. "We thought, why can't we do this? They said, 'There's no reason you can't.'" Lahmeyer was encouraged by Christina Walters, who leads the USDA's plant germplasm research unit at Fort Collins. Walters is anxious to get as many plants as she can cryopreserved and stored before they disappear completely.

A page from a set of binders that records every plant that has ever been grown in The Huntington's gardens. Photograph by Kate Lain.
"There's always something coming up and you were wishing you could buy more time," says Walters, who works in collaboration with botanical gardens to cryopreserve plant material they ship to her. But she's even happier to see The Huntington start its own program.
"There are precious few researchers doing this," says Walters. "We can't do all the things necessary unless we play together." Lahmeyer had a good start because the garden had a tissue culture lab, built in 2000, for propagating large numbers of plants. The lab included the sterile workplace that was needed; it was not difficult to add in liquid nitrogen and freezers. But the lab was missing one critical thing: a cryopreservationist. Through a global call, Lahmeyer found Folgado.
Folgado, a 35-year-old native of Spain, is energetic, down to earth, and fiercely devoted to her field, which she calls "cryo." The ninth of 12 children, Folgado's first botany lessons came from her parents, who grew apples, pears, potatoes, and wheat on a small farm in the mountains of Zamora, a Spanish province near Portugal known for deep-red Toro wines.
Folgado excelled in biology and attended the University of Oviedo. She encountered cryopreservation during a summer course, volunteered to join a lab, and quickly took to the painstaking work on hop plants even as fellow students quit in frustration. "It's not so easy," she admits. "You have to love it." She does. Folgado thrives on laboratory bench work; she routinely puts in long days and calls it "playing." She's adored by colleagues, who say they love that she's so self-effacing and doesn't take herself too seriously. When asked what she likes about working with plants, she simply answers: "They never complain."
One reason for Folgado's success here is her training. She did her graduate work in Belgium at the University of Leuven with Bart Panis, a legend in the world of cryopreservation for work on simplifying critical techniques and using them to store hundreds of species of banana.

Left to right: Raquel Folgado sits at a microscope, under sterile conditions, and extracts tissue located in what is known as the shoot apical meristem of an Aloe fievetii (photograph by Kate Lain); extracted A. fievetii tissue viewed under the microscope's lens (photograph by Raquel Folgado); Folgado places the plant tissue in a micro-droplet of solution on a small rectangle of aluminum foil (photograph by Kate Lain).
Cryopreservation of plants is very tricky: it requires freezing plant tissue, which is full of water, without letting destructive ice crystals form. To start a freezing protocol, Folgado must sit at a microscope, under sterile conditions, and deftly tease out the tiniest bit of plant: a one-millimeter- by-one-millimeter segment barely visible to the naked eye. The tissue, located in what is known as the shoot apical meristem, is hidden between tiny leaves and contains special cells, akin to stem cells, that can regenerate an entire new plant.
The work takes patience, good hands, and very good eyesight. A single experiment may require more than 60 samples. Folgado often has to stop to rest her eyes. Yet she must move quickly so that the plant tissue doesn't dry out.
Then she must remove as much water as possible without killing the plant tissue. To do this, Folgado tinkers with solutions—elixirs of vitamins, sugars, antioxidants, and antifreezes (such as glycerol)—that replace water in plant cells with fluids less likely to form ice. It's a delicate dance: the best antifreeze agents are usually toxic to plants. Not leaving tissue in solution long enough may mean it won't freeze well. But leaving it in too long could kill it.
Once the sample has had much of its water replaced, Folgado must cool it as quickly as possible so ice does not have time to form. Folgado uses a technique called droplet vitrification. She places the plant tissue in a micro-droplet of solution on a small rectangle of aluminum foil. The technique freezes the sample at an ultrafast rate of more than 260 degrees Fahrenheit per second.
Working very carefully—"otherwise your fingers will be cryopreserved," she notes—she then places the aluminum into liquid nitrogen. "This is the dangerous part," she says. "Ice will break the cells." If it works, the sample will cool so quickly that ice crystals do not form. Instead, the fluid will vitrify, or turn into a non-crystalline glass. The tissue should then remain viable, perhaps indefinitely, until it is thawed and revived.
The field of plant cryopreservation has come a long way since its birth in the mid-1950s. For decades, scientists had to start from scratch and create a detailed freezing procedure for each plant—work that could take years. The techniques Folgado has learned are robust enough to work fairly well on a wide variety of plants—but the process must still be fine-tuned for new plants; this process can take months of experimenting. (Folgado's experiments at The Huntington have already yielded some successful new freezing protocols.) Even after the protocols are developed, though, systematically storing large numbers of plants can take years, Panis notes.
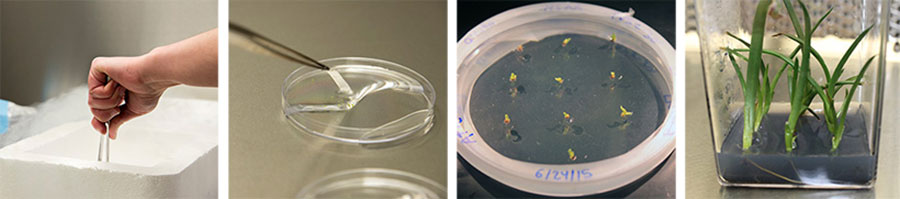
Left to right: Folgado lowers the tissue on the foil into liquid nitrogen (photograph by Kate Lain); the frozen bits of tissue thaw in a warming solution (photograph by Kate Lain); A. fievetii growing successfully three weeks after thawing (photograph by Raquel Folgado); A. fievetii thriving six months after thawing (photograph by Raquel Folgado).
"My dream is to develop a kind of Svalbard Number Two for cryopreserved material," says Panis, who now works for Bioversity International, a global group dedicated to preserving agricultural and tree biodiversity. "Of course, this means that people overseeing individual collections have to do a lot of work."
Folgado was finishing her doctoral work on potatoes when she learned that The Huntington wanted to cryopreserve a number of plants with no established protocols. It was a huge scientific challenge—to tease out the secrets of diverse and threatened plants. Though still at the start of her career, Folgado hopes to make contributions in her field by using experiments and knowledge of plant physiology to create simple and robust freezing protocols that can be widely used.
"The reason I came was the project," Folgado says. "I'm really in love with this work. I believe it can make a difference."
Folgado arrived at The Huntington in August 2014. Her first cryopreservation success came within a year with Aloe fievetii, an orange and red firecracker of a plant that was already being grown in the lab. She was able to freeze and then successfully regrow new plants from 70 percent of samples—a high success rate.
Aloes were chosen as a starting point because they are a conservation priority and a core collection at The Huntington. Of the more than 500 species of aloes known worldwide, The Huntington has housed 325 of them. The protocol Folgado developed for A. fievetii seems to work handily on other aloes, which should speed future aloe conservation projects. She is also working on cryopreserving aloe seeds for projects in which growing plants from seeds is a better strategy.
One plant in dire need of help is Aloe bowiea, which grows hidden among grasses and has been displaced in its South African habitat by the spread of shantytowns. Lahmeyer and Folgado are seeking partners in South Africa who can learn the aloe cryopreservation techniques Folgado has developed and use them to save and reintroduce the succulents. They hope to create similar partnerships with Mexican botanists to help preserve agaves—plants that flower so rarely that being able to create new plants from frozen tissue is critical.
This "exporting" of knowledge, Lahmeyer says, is a way of giving back to the foreign countries and wild landscapes where the early stewards of The Huntington once did so much collecting.
Folgado has also succeeded in cryopreserving the seed from a number of species of cactus—the garden's renowned desert collection holds samples of two-thirds of the world's 1,500 known cactus species—and preserving this germplasm would be a large contribution to the conservation of this threatened plant family. Folgado is very curious about why cactus seeds tolerate liquid nitrogen so well and thinks they may hold some physiological secrets, perhaps related to their drought tolerance, that could be key to improving freezing success rates in less hardy plants.

Aloe fievetii in bloom. Photograph by John Trager.
Another conservation target centers on magnolia trees: of 230 known species, 200 are endangered. But the project has been a challenge since many wild magnolias, rarely studied, do not have protocols for being grown in sterile lab conditions. "At the beginning, we killed a lot of material," Folgado confesses. She is working with Panis on the magnolias; after a few rounds of experiments, they have successfully grown a number of species in the lab and hope the work will lead to a way to speed cryopreservation of other wilder and little-studied species.
The fact that some plants prove more troublesome in the lab comes as no surprise to Valerie Pence. An expert on mosses and ferns, Pence has spent nearly three decades leading an innovative plant cryopreservation program at the Cincinnati Zoo and Botanical Garden. (Along with Omaha's Henry Doorly Zoo and Aquarium, the Cincinnati Zoo is one of a few smaller institutions with cryogenic programs. Zoos have been leaders because liquid nitrogen has long been used for in-vitro fertilization programs.)
Pence fears that plants that are more time-consuming to work with—including many of Folgado's targets—could easily be left out of conservation efforts.
"If it costs so much to deal with them and you could bank 10–20 other species at the same cost, will they get preserved?" Pence asks. "There's so much to be done, and the resources are limited."
Another plant that's proven difficult is the avocado tree. Avocados are a priority because The Huntington was home to one of California's first commercial avocado groves (in what is now the visitor parking lot). Many avocado cuttings from the garden have not been growing well in tissue culture, perhaps because of fungi and pest burdens. But Folgado is not giving up. Such challenges seem to drive her even more. To speed her efforts, Folgado has been working long distance with a team in Queensland, Australia, where avocado is also a commodity and where the plants grow better in lab conditions. She helps with experiments there via Skype.
In addition to these conservation efforts and international collaborations, the cryopreservation project has one very long-term goal: to preserve The Huntington's collection for the future in dewars, or double-walled flasks, of liquid nitrogen. This frozen garden could offer a way to propagate critical plants not only into new locations and habitats, but also into the future as well.
"If I could have saved seeds or tissue from every plant of importance we ever got," Folsom muses, patting the cover of one of his thick black binders, "this would not be a book of memories. This would be a library, and I could say, 'Next year, let's bring this plant out.' In 100 years, it would be very sad if someone has not done this."
Folgado shares Folsom's dream of a parallel frozen garden. And she loves being able to stroll out of her office to observe plants and choose new species to work with. Her problem is that there are so many plants and so little time. The urgency keeps pushing her forward—one plant at a time. There are cycads, too, of course. And rare orchids. "The camellias," she adds, "are also waiting for us."
Usha Lee McFarling is a Pulitzer Prize–winning freelance writer based in South Pasadena, Calif.